The Board of Appeal of the European Union Intellectual Property Office (“EUIPO”) is required, upon request, to review decisions made by the Cancellation Division in their entirety, to reassess the dispute in its entirety and, finally, to provide sufficient reasons for its own decision. A recent decision of the European General Court provides new guidance on the scope of these obligations.
The case in question
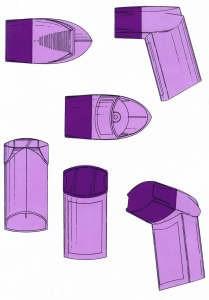
The decision of the European General Court was preceded by an application for a declaration of invalidity of a 3D color trademark registered by Glaxo (“the Applicant”) since 2001 for various pharmaceutical products in goods classes 5 and 10:
The Cancellation Division of EUIPO granted the application for a declaration of invalidity on the basis of Article 52(1)(a) in conjunction with Article 7(1)(b) of Regulation (EC) 2017/1001 due to a lack of distinctiveness of the mark. A subsequent appeal by Glaxo to the Board of Appeal of EUIPO was dismissed on the same grounds.
Glaxo subsequently applied to the European General Court for an annulment of the decision of the First Board of Appeal of the EUIPO, claiming in particular:
- Violation of Article 41 paragraph 2 CFR:
The Board of Appeal did not examine the original distinctiveness of the mark, which results from the combination of the different elements (shape and color). Thereby, the “right to good administration” according to Article 41(2) of the Charter of Fundamental Rights of the EU (CFR) was violated.
- Infringement of Article 7(1)(b) of Regulation (EU) 2017/1001:
According to the Applicant, the Board of Appeal’s statement of reasons for its decision contains contradictions with regard to the assessment of distinctive character.
The decision of the European General Court
The European General Court allowed the action and annulled the decision of the Board of Appeal of EUIPO on the following grounds:
Examination of the original distinctive character
The European General Court agreed with Glaxo that, although the Board of Appeal had recognized that the mark at issue was a complex mark characterized by its shape and color scheme, those elements of the mark had been considered only separately and not as a whole.
With regard to the shape, the Board of Appeal limited itself to stating that it had been protected by a patent in the 1950s. In the Board of Appeal’s view, the coloration was descriptive of the active components and of the purpose and properties of the medicinal product.
It follows, in the view of the European General Court, that the Board of Appeal was in breach of its duty to carry out an overall assessment of the elements of the challenged mark, because: “the mere fact that each element of the mark is devoid of distinctive character in itself does not mean that their combination cannot have distinctive character”.
Lack of justification
The European General Court stated that the Board of Appeal was obliged under Article 94 (1) sentence 1 of Regulation (EU) 2017/1001 to disclose a clear and unambiguous statement of reasons for its decision. Such a statement of reasons was not dispensable just because it confirmed the result of the decision of the Cancellation Division.
There was also no full confirmation in the present case. First, it is not clear from the contested decision that the Board of Appeal referred to the decision of the Cancellation Division. Furthermore, both entities had reached the same conclusion on the basis of significantly different reasons.
The Cancellation Division took the view that the coloration was generally a common feature of the product category concerned and would therefore be perceived by the relevant public as a common component of the goods, but precisely not as an indication of origin. On the other hand, the Board of Appeal considered that the coloration was descriptive in nature, since it had an informative function with regard to the active components and the purpose and properties of the medicinal product.
Contradictory justification
Furthermore, the European General Court found the Board of Appeal’s reasoning to be partially contradictory:
The Board of Appeal found, on the one hand, that the Applicant had not produced any evidence against the existence of an inherent distinctive character in the invalidity proceedings. On the other hand, it reached the opposite conclusion on the basis of the same evidence, attributing a purely informative function to the color scheme of the goods in question.
Moreover, the Board of Appeal first found that in the pharmaceutical industry, periods of 10 years are already long enough for the perception of the relevant public to change significantly in line with new trends. Subsequently, however, it applied principles for determining the descriptive character of the color scheme which were developed in a decision of 2020 and thus at a point in time which is almost 14 years apart from the point in time relevant for determining distinctiveness.
In the European General Court’s view, these contradictions in the Board of Appeal’s reasoning amount to a failure to state reasons.
Practice note
While Glaxo was able to avoid the declaration of invalidity of its trademark, this ruling could well be difficult to digest for the other parties involved, because the reversal of the appeal decision is ultimately the result of inaccuracies in the reasons for the decision and not necessarily an erroneous decision in the substantive legal sense. Whether the 3D color mark in its entirety actually has distinctive character has not been examined atWith regard to legal protection and legal certainty, this decision is to be welcomed. The detailed statement of reasons for the decision of the Board of Appeal is intended, on the one hand, to enable the party concerned to understand the reasons for the measure and, on the other hand, to enable the courts of the European Union to review the legality of the decisions. Notwithstanding the fact that it confirmed the Cancellation Division’s decision in the result, the Board of Appeal was not allowed to shorten either the examination or its reasoning in the light of the Cancellation Division’s discussions that had already taken place. all so far, so that this controversial question still remains outstanding and must now be assessed again by the Board of Appeal.
With regard to legal protection and legal certainty, this decision is to be welcomed. The detailed statement of reasons for the decision of the Board of Appeal is intended, on the one hand, to enable the party concerned to understand the reasons for the measure and, on the other hand, to enable the courts of the European Union to review the legality of the decisions. Notwithstanding the fact that it confirmed the Cancellation Division’s decision in the result, the Board of Appeal was not allowed to shorten either the examination or its reasoning in the light of the Cancellation Division’s discussions that had already taken place.
In view of the high demands placed on the Board of Appeal’s examination and statement of reasons for its decision, the recipient of an onerous appeal decision of the EUIPO should not hesitate to have it reviewed by a lawyer and, if necessary, by the courts.